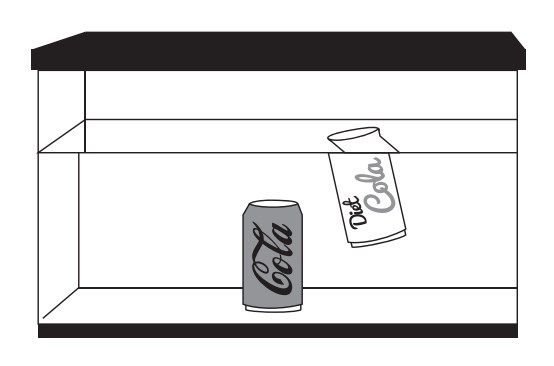
In this activity, students learn about the fundamental principle of density (amount of mass per volume) by comparing the buoyancy of two types of cola.
When you place a can of cola in water it will sink. A can of diet cola, however, will float. This shows that they have different densities.
The amount of aluminum in each can is the same, as is the amount of fluid (355 ml) and the amount of carbon dioxide. What varies between cola and diet cola is the sweetener. For example, a can of Coke Classic contains 39 grams of sugar and a can of Diet Coke contains 0.1 grams of aspartame.
It takes 390 times more sugar to make a drink taste as sweet as a drink sweetened with aspartame. This additional sugar makes the regular cola more dense.