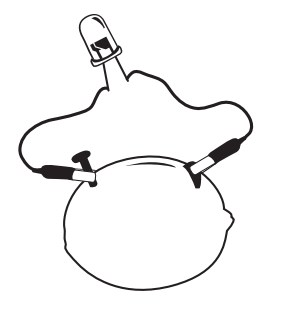
You can make a battery using a piece of fruit?
Yes, technically, but not a very strong one!
The source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon.
The citric acid of the lemon reacts with the zinc and loosens electrons. Copper pulls electrons more strongly than zinc, so loose electrons will move towards the copper when the electrodes are connected by wires. Moving electrons are called an electric current, which is what lights up the bulb.
Teacher Tip:
This is a classic electricity activity, but it can be very frustrating if you don't have the right equipment. We recommend having a multimeter or voltmeter on hand to test the voltage.