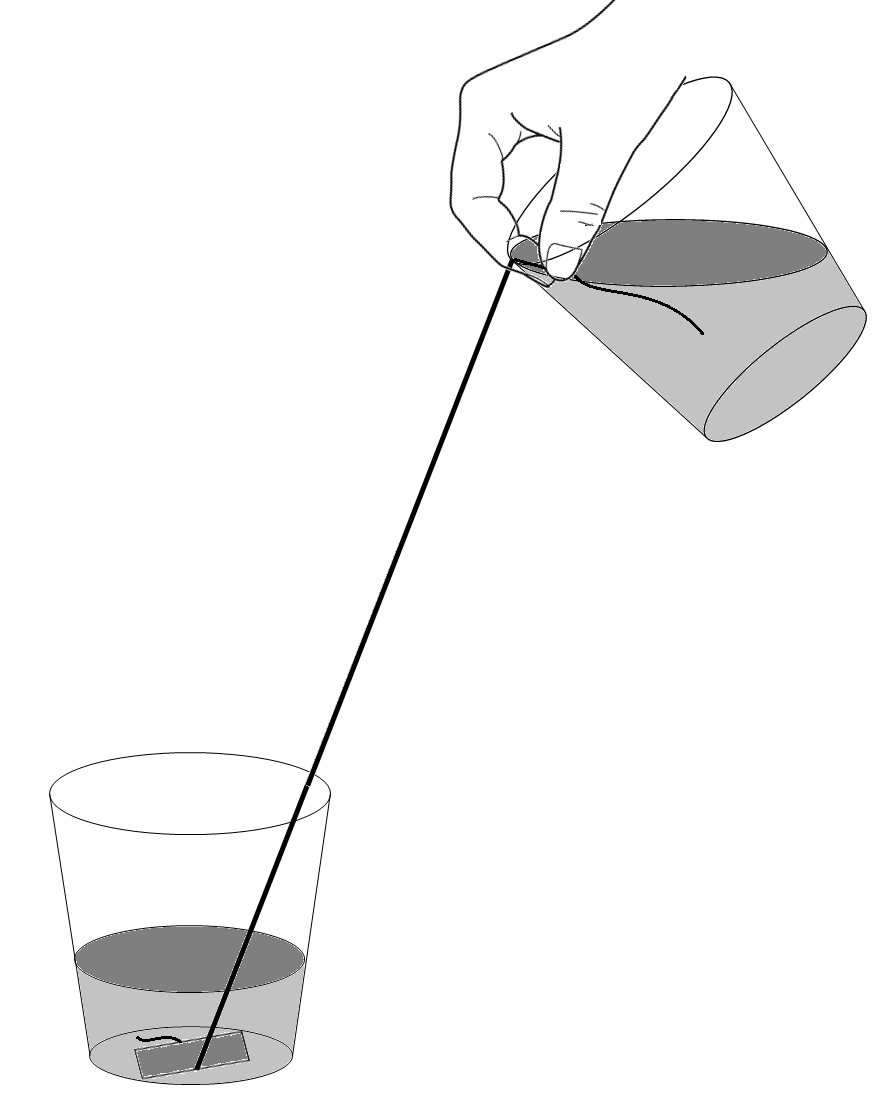
This demonstration uses simple materials to introduce the adhesive and cohesive properties of water.
The two forces at play in this 'trick'. One is adhesion (the attraction between the water and the surface of the string), and the other is cohesion (the attraction of water to itself). Together, they are stronger than gravity, which pulls the water toward the ground.
Adhesion and cohesion attractive forces occur because water molecules are polar. Each molecule of water has two atoms of hydrogen and one atom of oxygen (H2O). Because the (slightly positive) hydrogen atoms from one molecule will attract the (slightly negative) oxygen atoms from another, the molecules tend to stick together and to other surfaces/substances.
The properties of adhesion and cohesion can be easily observed by watching raindrops on a windowpane. Adhesion holds the drops to the glass. Even if the window is tilted forward, some drops will cling to the underside of the pane. Cohesion can be seen if you trace the path of drops down the pane. Drops close to one another will be drawn together by cohesion, forming larger drops. Drops are more likely to join together than split apart. Splitting a water drop requires some energy or change to loosen the bonds that hold the molecules together.