In this activity, students predict then examine the density of common household liquids.
Dense liquids will sink to the bottom, while less-dense liquids move above them.
In this activity, students predict then examine the density of common household liquids.
Dense liquids will sink to the bottom, while less-dense liquids move above them.
Predict, test and explain the relative densities of common liquids.
Per Demo:
1 tall, clear cup
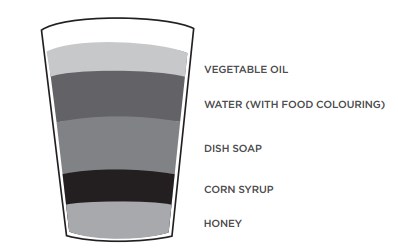
3 liquids of different densities (choose from): honey, corn syrup, dish soap, water with a drop of food colouring, vegetable oil, food colouring
Teacher Tips
Pour each layer slowly, because mixing can make it more difficult to see the intended result. Start with the densest liquid, then add liquids with lower densities. Tilt the cup and pour onto the side of the cup to cause the least disturbance to the liquids.
About the sticker
Survivors
Artist: Jeff Kulak
Jeff is a senior graphic designer at Science World. His illustration work has been published in the Walrus, The National Post, Reader’s Digest and Chickadee Magazine. He loves to make music, ride bikes, and spend time in the forest.
About the sticker
Egg BB
Artist: Jeff Kulak
Jeff is a senior graphic designer at Science World. His illustration work has been published in the Walrus, The National Post, Reader’s Digest and Chickadee Magazine. He loves to make music, ride bikes, and spend time in the forest.
About the sticker
Comet Crisp
Artist: Jeff Kulak
Jeff is a senior graphic designer at Science World. His illustration work has been published in the Walrus, The National Post, Reader’s Digest and Chickadee Magazine. He loves to make music, ride bikes, and spend time in the forest.
About the sticker
T-Rex and Baby
Artist: Michelle Yong
Michelle is a designer with a focus on creating joyful digital experiences! She enjoys exploring the potential forms that an idea can express itself in and helping then take shape.
About the sticker
Buddy the T-Rex
Artist: Michelle Yong
Michelle is a designer with a focus on creating joyful digital experiences! She enjoys exploring the potential forms that an idea can express itself in and helping then take shape.
About the sticker
Geodessy
Artist: Michelle Yong
Michelle is a designer with a focus on creating joyful digital experiences! She enjoys exploring the potential forms that an idea can express itself in and helping then take shape.
About the sticker
Science Buddies
Artist: Ty Dale
From Canada, Ty was born in Vancouver, British Columbia in 1993. From his chaotic workspace he draws in several different illustrative styles with thick outlines, bold colours and quirky-child like drawings. Ty distils the world around him into its basic geometry, prompting us to look at the mundane in a different way.
About the sticker
Western Dinosaur
Artist: Ty Dale
From Canada, Ty was born in Vancouver, British Columbia in 1993. From his chaotic workspace he draws in several different illustrative styles with thick outlines, bold colours and quirky-child like drawings. Ty distils the world around him into its basic geometry, prompting us to look at the mundane in a different way.
About the sticker
Time-Travel T-Rex
Artist: Ty Dale
From Canada, Ty was born in Vancouver, British Columbia in 1993. From his chaotic workspace he draws in several different illustrative styles with thick outlines, bold colours and quirky-child like drawings. Ty distils the world around him into its basic geometry, prompting us to look at the mundane in a different way.