In this activity, students describe the effects of an acid on an eggshell. The reaction of the eggshell in vinegar is an acid-base reaction. When you submerge an egg in vinegar, the shell dissolves, leaving the inner semi-permeable membrane intact.
Vinegar (acid) breaks apart the solid calcium carbonate crystals (base) in the eggshell into their calcium and carbonate parts. The calcium ions stay dissolved in the vinegar (calcium ions are atoms that are missing electrons), while the carbonate goes on to make carbon dioxide — the bubbles that you see.
The acidic vinegar leaves the membrane that lines the inside of the shell intact. Some of the vinegar permeates the membrane due to osmosis, which is why the egg swells. If you shake the egg, you can see the yolk sloshing around in the white. If the membrane tears, the contents will spill out just the same as with any raw egg, only now they have been "pickled" in the vinegar.
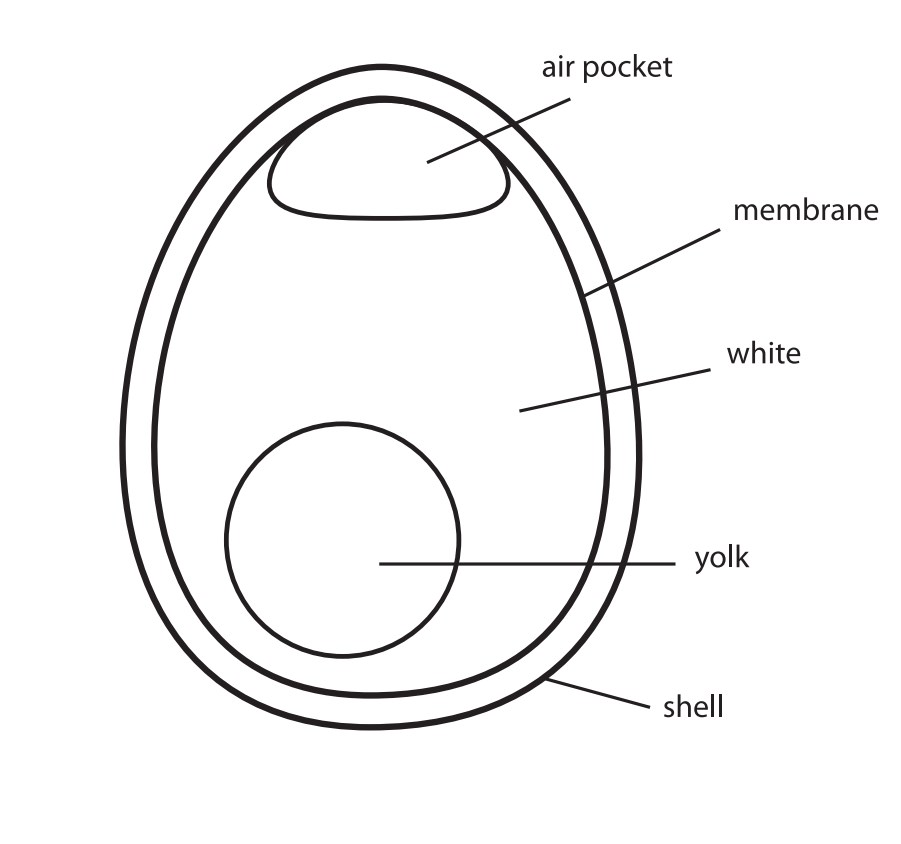
Teacher Tip: Younger students may think that the outer shell has "transformed" into the membrane. Remind them that the outer shell and the inner membrane are two completely different layers. You can crack a raw egg to point out the layers.


 copy.jpg)